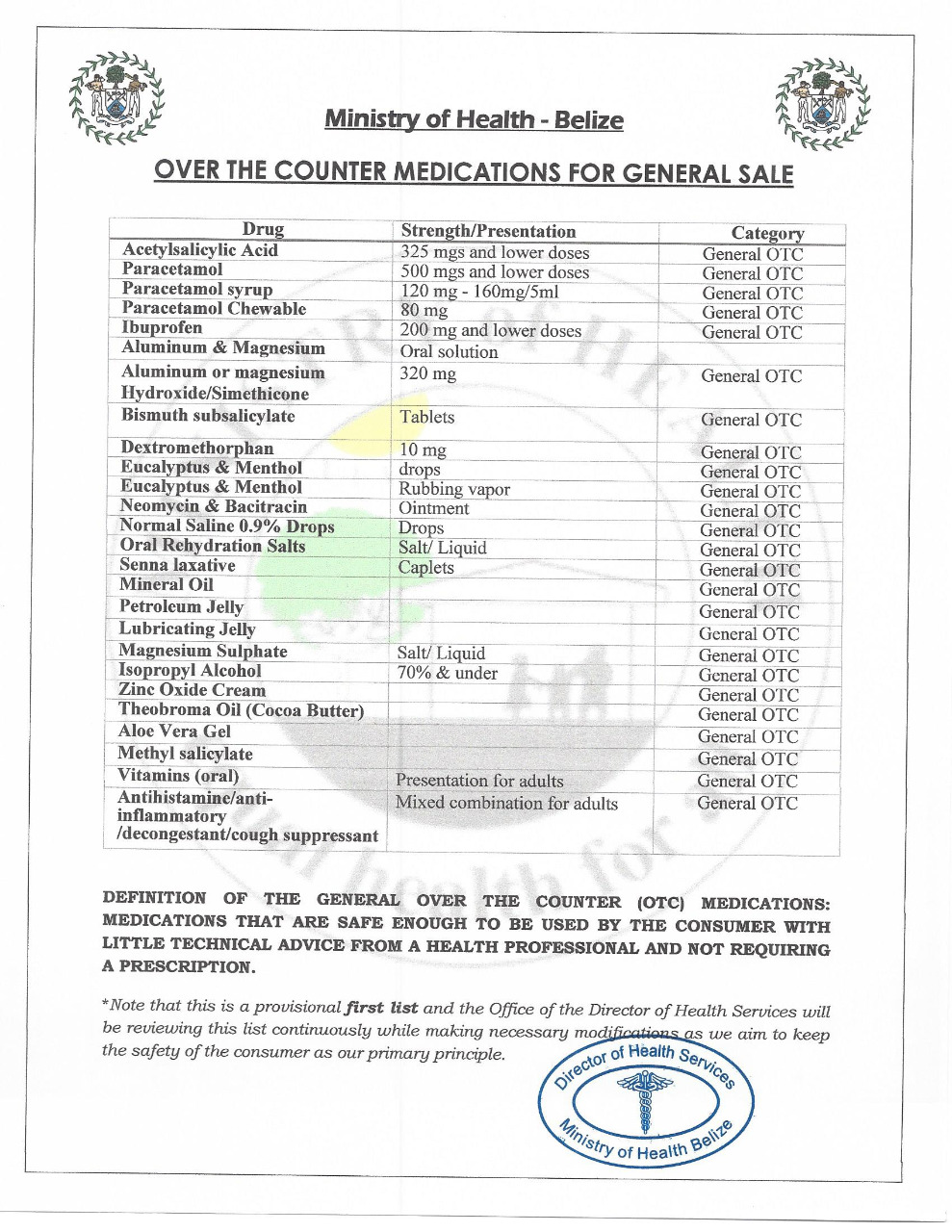
The Unit of Medicines and Health Technologies of PAHO / WHO is pleased to invite you to:
Webinar: Use of REDCap for the improvement of health care processes (in Spanish)
Friday, February 16, 2018, 10:00 a.m. of Washington DC, United States
REDCap is a web software created by Paul Harris and colleagues at Vanderbilt University and designed for data management in Translational Research. With more than 10 years of development it offers a large number of features that make it attractive for researchers (multi-projects, security, surveys, randomization, branches, blind, automatic emails, access groups, user profiles, among many others). All this has led REDCap to be adopted by more than 2700 institutions, reaching almost 700,000 users in 117 countries, and has been cited in more than 4700 publications. Although the initial intention was to develop a software for Research, the use of this tool for management and operation is being increasingly validated worldwide. This presentation aims to convey the success stories of the use of REDCap for both clinical and administrative management of health care.
Cristóbal Carvajal is a surgeon, graduated from “Universidad del Desarrollo”, Santiago Chile. 5 years ago, she works in the Biomedical Informatics Department at “Clínica Alemana de Santiago”, and since 2015 she is in charge of the Clinical Data Unit. The development of its activity has been based on innovation and creative solution of problems through the use of information, so it has specialized in the modeling and data capture in different scenarios. He recently completed a stay at Quality & Improvement at the Children’s Hospital of Philadelphia, USA, which has allowed him to complement his vision of data analysis with quality aspects. Dr. Carvajal is an advanced user of the REDCap software and a pioneer in the use of this tool for purposes other than research, which has allowed him to capture information and optimize various processes in multiple scenarios. He has presented his experience in various congresses in Chile, Argentina and USA. He is an active participant in the REDCap community and a member of the Spanish-speaking group. In addition, from 2013 to the present, he works as a clinician in an Emergency Service of the “Mutual de Seguridad” (which covers accidents at work and occupational diseases).
Diego Macías Saint-Gerons has a degree in pharmacy from the University of Salamanca, a Master’s Degree in Quantitative Methods of Research in Epidemiology from the Autonomous University of Madrid and a PhD in Health Sciences from the Complutense University of Madrid. He is the author of numerous scientific articles on the use of drugs and their effects. He has worked at the University of Valladolid, the Spanish Agency for Medicines and Health Products (AEMPS), and as an expert of the European Medicines Agency (EMA). He is currently working as an international consultant (IPC) in the Medicines and Health Technologies Unit of PAHO / WHO.
Access will be provided through: https://paho.webex.com/paho/onstage/g.php?MTID=e11f25011f597fc2e8ea93b378557adc4
Additionally, it can be accessed by telephone through the United States line: + 1-415-655-0002 by dialing the access code 642 964 895
We hope count with your participation.
HSS / MT