XIII Encuentro Regional de la Red de Evaluación de Tecnologías en Salud de las Américas
Washington DC, 22 de noviembre de 2022 (OPS)– Los días 7, 8 y 9 de noviembre de 2022 se llevó a cabo el XIII Encuentro Regional de la Red de Evaluación de Tecnologías en Salud de las Américas (RedETSA) en la ciudad de Brasilia, Brasil. Representantes de 18 países debatieron sobre el proceso de evaluación de tecnologías sanitarias (ETS) en todas las etapas del ciclo de vida de las tecnologías, para afrontar los retos de su incorporación en los sistemas de salud. El evento se organizó en conjunto al III Congreso de la Red Brasilera de Evaluación de Tecnologías en Salud (Rebrats).
Participaron de la apertura del evento Marcelo Queiroga, Ministro de Salud de Brasil; Socorro Gross Galiano, Representante de la oficina de país de OPS/OMS en Brasil; Sandra Barros, secretaria de ciencia, tecnología, innovación e insumos estratégicos en salud; Vania Canuto, directora del Departamento de gestión e incorporación de tecnologías en salud; y Alexandre Lemgruber, asesor regional en gestión de tecnologías sanitarias de la OPS.
Como parte del Encuentro, se desarrollaron jornadas científicas abiertas y organizadas conforme a las diferentes etapas del ciclo de vida de las tecnologías sanitarias (regulación-evaluación-incorporación-uso-monitoreo). Participaron 311 personas de manera presencial y 228 personas de forma virtual gracias a la transmisión directa del evento.
Mayores informaciones disponibles por el enlace https://www.paho.org/es/noticias/22-11-2022-xiii-encuentro-regional-red-evaluacion-tecnologias-salud-americas
La OPS pone el tratamiento para la COVID-19 a disposición de 16 países
Washington, DC, 21 de diciembre de 2022 (OPS) – En un esfuerzo por aumentar el acceso equitativo a los tratamientos para la COVID-19, la Organización Panamericana de la Salud (OPS) entregó más de 11.000 viales de un medicamento para mejorar el tratamiento de pacientes gravemente enfermos de COVID-19 en 15 países de América Latina y el Caribe.
El medicamento, tocilizumab, ha demostrado reducir la mortalidad en pacientes hospitalizados con COVID-19 grave o crítico, que se deterioran rápidamente o necesitan mayores niveles de oxígeno, y que tienen una respuesta inflamatoria significativa.
La compra, con valor de más de 2 millones de dólares, fue realizada por la OPS con el apoyo del Gobierno de Estados Unidos.
Fuente:
WHO urges action to protect children from contaminated medicines CRM:0557163
Geneva, 23 January 2023 – WHO is releasing an urgent call to action to countries to prevent, detect and respond to incidents of substandard and falsified medical products.
Over the past four months, countries have reported on several incidents of over-the-counter cough syrups for children with confirmed or suspected contamination with high levels of diethylene glycol (DEG) and ethylene glycol (EG). The cases are from at least seven countries, associated with more than 300 fatalities in three of these countries. Most are young children under the age of five. These contaminants are toxic chemicals used as industrial solvents and antifreeze agents that can be fatal even taken in small amounts, and should never be found in medicines.
Based on country reports, WHO has issued three global medical alerts addressing these incidents. The Medical Product Alert N°6/2022 on 5 October 2022 focused on the outbreak in the Gambia, Medical Product Alert N°7/2022 on 6 November 2022 focused on Indonesia, and Medical Product Alert No1/2023 on 11 January 2023 focused on Uzbekistan.
WHO’s medical product alerts were rapidly disseminated to the national health authorities of all 194 WHO Member States. These medical product alerts requested, inter alia: (a) the detection and removal of contaminated medicines from circulation in the markets, (b) increased surveillance and diligence within the supply chains of countries and regions likely to be affected, (c) immediate notification to WHO if these substandard products are discovered in-country; and otherwise inform the public of the dangers and toxic effects of the substandard medicines at issue.
Since these are not isolated incidents WHO calls on various key stakeholders engaged in the medical supply chain to take immediate and coordinated action.
For more information go to WHO urges action to protect children from contaminated medicines
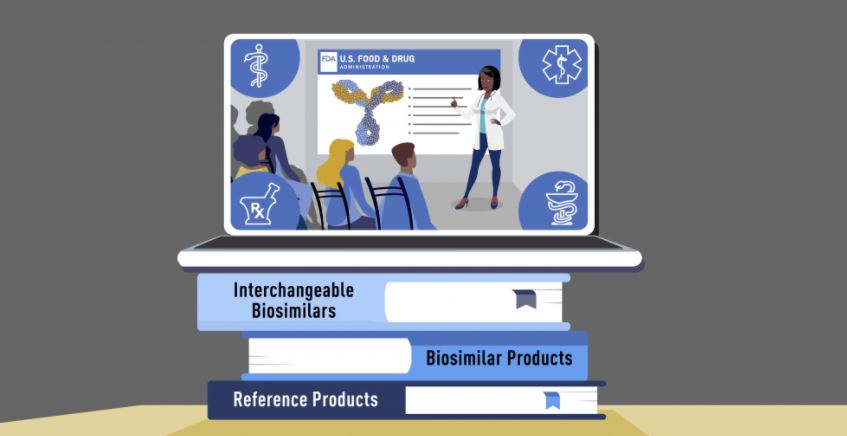
Biosimilar and interchangeable biosimilar products – USFDA Curriculum Materials for Health Care Degree Programs
Extensão de prazo para 15 de outubro de 2021
Call for expression of interest to contribute to the value chain and supply of reagents for the sustainable manufacturing of a COVID-19 and other mRNA vaccines in the Americas [Deadline extension from 17 September to 15 October 2021]
For the successful establishment of mRNA vaccine manufacturing capacity globally, the World Health Organization (WHO) has launched a new initiative to support transfer of technology to produce mRNA vaccines in low- and middle-income countries (LMICs) (1). The initiative will ensure that all WHO regions will be able to produce vaccines as essential preparedness measure against future infectious threats.
Under this initiative, WHO and the Pan American Health Organization (PAHO) are working together to establish mRNA vaccine manufacturing capacities in LAC. Currently, there is a limited number of global manufacturers able to supply all necessary reagents and/or starting materials (2) needed for manufacturing mRNA vaccines. To ensure sustainability and independence of vaccine production in the Region, access to reagents that are critical to the value chain is fundamental. Hence PAHO/WHO is launching a call for expression of interest to manufacturers in the Region of the Americas that wish to become part of a regional consortium that will ensure that mRNA vaccines can be sourced from starting materials and preparations (including seed lots, cell banks and intermediates) to the finished product.
Through this new initiative, PAHO and WHO will facilitate the establishment of a Regional consortium and support of a comprehensive technology transfer. Ideally, the group of manufacturers that come together for this initiative will include representatives from different geographical areas in the Americas and work towards an integrated regional value chain that will provide sustainability and ease the dependence on imported vaccines from outside the Region.
To support this activity, we are seeking expressions of interest from:
- Public or private manufactures of medical products (drugs, vaccines or drug substances) , and/or starting materials from the Region of the Americas, which could manufacture and supply one or more of the following:
- DNAse 1,
- T7 RNA Polymerase,
- RNase inhibitor,
- Guanyl Transferase,
- Pyrophosphatase,
- GTP,
- s-adenosyl methionine, and
- ribonucleotides.
Entities willing to be considered are invited to provide a brief summary that includes, at a minimum, the reagents and/or starting materials they wish to provide, a summary of their capacity, needs, and their interest in participating in this initiative.
Deadline submission
Deadline extension from 17 September to 15 October 2021
This information must be sent to:
MT@paho.org
(1). See Call for expression of interest to: Contribute to the establishment of a COVID-19 mRNA vaccine technology transfer hub (who.int)
(2). In accordance with WHO TRS Sixty-sixth report starting materials are: any substances of a defined quality used in the production of a pharmaceutical product but excluding packaging materials. In the context of biological products manufacturing, examples of starting materials may include cryo-protectants, feeder cells, reagents, growth media, buffers, serum, enzymes, cytokines, growth factors and amino acids.
OPAS seleciona centros na Argentina e no Brasil para desenvolver vacinas de mRNA contra COVID-19
Os selecionados foram o Instituto de Tecnologia em Imunobiológicos da Fundação Oswaldo Cruz (Bio-Manguinhos/Fiocruz) e a empresa biofarmacêutica Sinergium Biotech
Washington, DC, 21 de setembro de 2021 (OPAS) – A Organização Pan-Americana da Saúde (OPAS) anunciou a seleção de dois centros regionais para o desenvolvimento e produção de vacinas de mRNA na América Latina, na Argentina e no Brasil, a fim de enfrentar a COVID-19 e os futuros desafios de doenças infecciosas.
O Instituto de Tecnologia em Imunobiológicos da Fundação Oswaldo Cruz (Bio-Manguinhos/Fiocruz) foi selecionado como centro no Brasil. Este conta com uma longa trajetória na fabricação de vacinas e fez avanços promissores no desenvolvimento de uma vacina de mRNA inovadora contra a COVID-19.
A empresa biofarmacêutica privada Sinergium Biotech foi escolhida como centro na Argentina e fará parceria com a empresa de biotecnologia mAbxience – pertencente ao mesmo grupo – para desenvolver e fabricar os princípios ativos da vacina. Ambas as empresas têm ampla experiência na produção e desenvolvimento de imunizantes e outros produtos médicos biotecnológicos.
O anúncio foi feito por Soumya Swaminathan, cientista chefe da Organização Mundial da Saúde (OMS), e por Jarbas Barbosa, subdiretor da OPAS, durante um evento paralelo à 59ª reunião do Conselho Diretor da OPAS. O evento “Transferência de tecnologia para produção de vacinas de mRNA nas Américas” reuniu ministros da Saúde e autoridades dos países da região para discutir a produção de vacinas.
“Parabenizamos os dois centros selecionados”, afirmou Barbosa, acrescentando que “ainda há muito trabalho árduo pela frente, mas somos movidos pela convicção de que este esforço resultará em acesso oportuno e equitativo às vacinas em nossa região, que continua sendo a mais afetada por esta pandemia.”
A seleção é resultado de uma convocatória de manifestação de interesse promovida pela OMS em abril de 2021, na qual fabricantes e instituições de pesquisa públicas e privadas foram convidados a contribuir para o estabelecimento de centros de transferência de tecnologia para vacinas de mRNA contra a COVID-19 em economias emergentes. A iniciativa teve o apoio de parceiros globais da OPAS/OMS, como a Medicines Patent Pool.
A convocatória atraiu cerca de 30 manifestações de interesse de empresas e instituições científicas latino-americanas. Para garantir a sustentabilidade e aumentar ainda mais a capacidade regional, a OPAS lançou uma segunda chamada para manifestações de interesse em agosto de 2021. Esta convocação foi especialmente dirigida a fabricantes interessados em fazer parte de um consórcio regional para fornecer reagentes de grau farmacêutico e outros insumos para a produção de vacinas de mRNA.
A OPAS também apresentou recentemente a Plataforma Regional para o Avanço na Produção de Vacinas e outras Tecnologias de Saúde para COVID-19 nas Américas, que apoiará a colaboração entre países e agências para aplicar a capacidade de biomanufatura regional existente à produção de vacinas e outras tecnologias médicas. A plataforma se baseia no princípio de que a fabricação de produtos farmacêuticos deve ser regional e beneficiar todas as Américas. A distribuição das vacinas será realizada a todos os países pelo Fundo Rotatório da OPAS.
“Os atrasos na produção (de vacinas) fizeram com que muitos países (da região) continuassem esperando pelas doses que compraram meses atrás. A limitação no fornecimento de vacinas continua atrasando” a vacinação, disse a diretora da OPAS, Carissa F. Etienne, em seu discurso de abertura no evento. “Esta produção limitada e distribuição desigual de vacinas em face da assombrosa demanda dificultam nossa resposta à COVID-19 nas Américas. A vacinação em massa é fundamental” para controlar a pandemia, acrescentou.
Até o momento, as Américas foram a região mais atingida pela COVID-19 em todo o mundo, com 87,6 milhões de casos registrados e mais de 2,16 milhões de vidas perdidas. A distribuição da vacina continua desigual e poucos países da região alcançaram a meta de vacinação de 40% da população estabelecida pela OMS.
CONTATOS:
Sebastián Oliel: +1 202 316-5679
Ashley Baldwin: + 1 202 340-4025
Daniel Epstein: +1 301 219-2105
Nancy Nusser: + 410 934-9588