The FDA Adverse Event Reporting System (FAERS) Public Dashboard
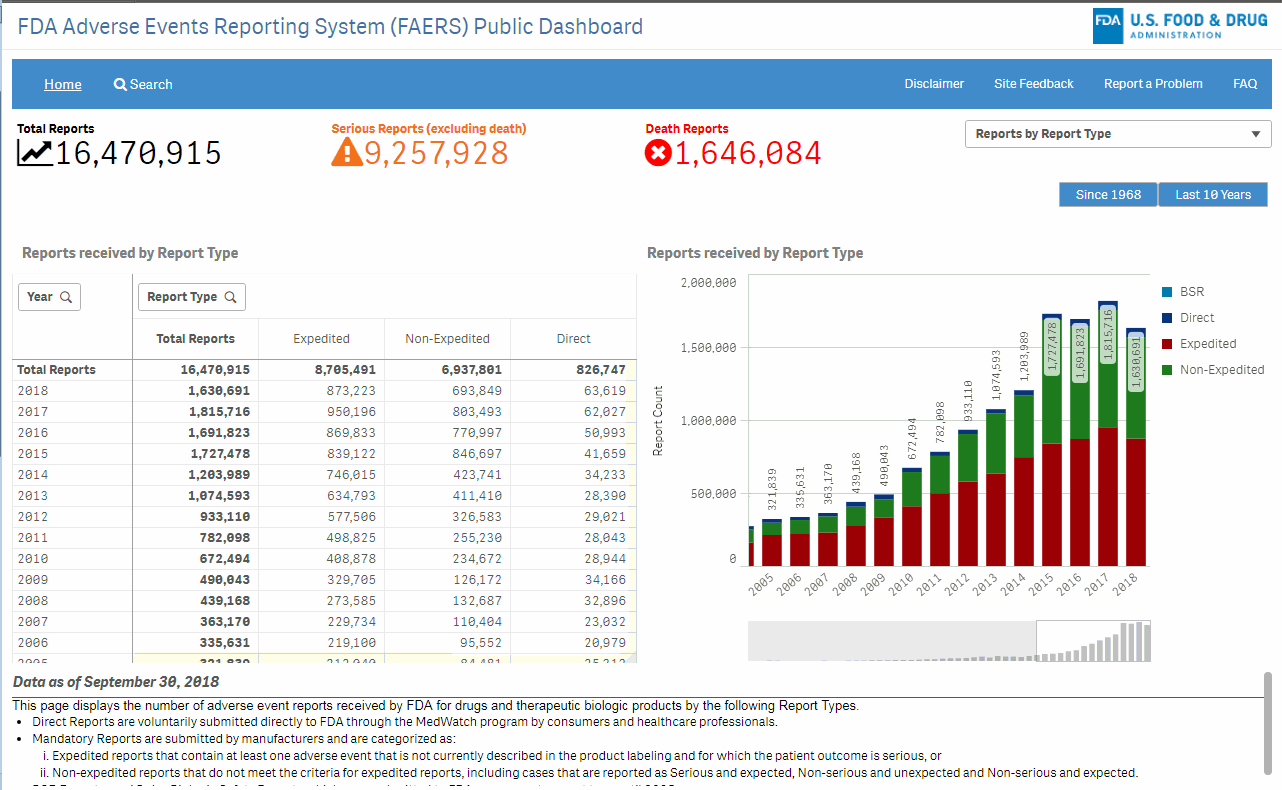
The FAERS Public Dashboard is a highly interactive web-based tool that will allow for the querying of FAERS data in a user friendly fashion. The intention of this tool is to expand access of FAERS data to the general public to search for information related to human adverse events reported to the FDA by the pharmaceutical industry, healthcare providers and consumers.
What is FAERS?
The FDA Adverse Event Reporting System (FAERS) is a database that contains adverse event reports, medication error reports and product quality complaints resulting in adverse events that were submitted to FDA. The database is designed to support the FDA’s post-marketing safety surveillance program for drug and therapeutic biologic products. The informatic structure of the FAERS database adheres to the international safety reporting guidance issued by the International Conference on Harmonisation (ICH E2B). Adverse events and medication errors are coded using terms in the Medical Dictionary for Regulatory Activities (MedDRA) terminology.
For more information go to https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm

